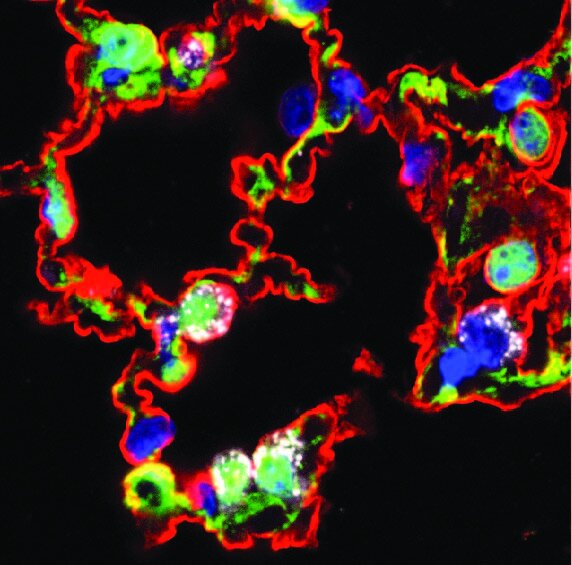
Lung progenitor cells in health and disease
As an adult regeneration lab in a pediatric hospital, the lab is ideally positioned to understand the intersection of pediatric and adult lung disease. Extensive human retrospective data implies a key relationship between pediatric respiratory insults, both before and after birth, and the risk of adult lung diseases including COPD and pulmonary fibrosis. The overall mechanisms underlying these risks are poorly understood.
The Zacharias Lung Lab uses the nonhuman primate Rhesus macaque, in collaboration with a multidisciplinary team at CCHMC and the California National Primate Research Center, to directly evaluate epigenetic and environmental mechanisms impacting lung development and regeneration. We have developed a large bank of non-human primate lung histology, single cell transcriptomics and epigenomics, and precision cut lung slices to directly assess mechanisms underlying lifelong lung health.
From Toth et al., 2022 Science Translational Medicine - read the paper
Developmental origins of adult lung diseases
Regeneration of the respiratory gas exchange interface after injury is a complex process requiring coordination of multiple cell types to rebuild the alveolar niche. Alveolar type 2 (AT2) cells have long been known to drive lung regeneration, but the mechanisms underlying this function remain largely undefined. During his post-doc, Will identified a Wnt-responsive alveolar type 2 cell called alveolar epithelial progenitor cells (AEPs), which are highly enriched for progenitor function and quite distinct from other AT2 cells in epigenome organization.
Now, the Zacharias lab studies the determinants of the AEP cell state, the transcriptional regulatory logic underlying their activity, and upstream mechanisms which promote AEP survival or expansion. Our hope is that understanding this cell state in detail will provide opportunities to develop new therapies to promote lung regeneration in patients.
From Zacharias et al., 2018 Nature - read the paper
Building complex lung organoid cultures
Alveolar epithelial progenitors (AEPs) provide an ideal starting material for adult lung organoids, and the lab has built a pipeline for creating large, clonal, complex lung organoids that contain the major epithelial cell types in the alveolus. These organoids provide an ideal model for studying the dynamics of lung epithelial regeneration in 3D and in real time. Using a combination of FACS, whole-mount immunohistochemistry, and viral-based gene knockouts, we are working toward characterizing AEP behavior in a dish.
The lab is also focused on the future of lung organoid cultures - for drug screening, infection modeling, and genome editing. We are currently working on adding other immune, stromal, and endothelial cells which inhabit the alveolus in vivo to build a true “alveolus in a dish” for disease modeling and basic discovery.
From Toth et al., 2023 Nature Comm - read the paper
Single cell transcriptomics and epigenomics across lung health and disease
Single cell technologies have been a major advancement in understanding complex organs like the lung, and our lab is fortunate to participate in the LungMap consortium funded by the NHLBI. With our close collaborator, the Miraldi lab, we have focused on moving beyond transcription to integrated analysis of the regulatory networks that underlie lung epithelial biology. Focusing on these networks provides mechanistic insight at a cell by cell level, allowing identification of new opportunities to improve the development and regeneration of the lung.
From Toth et al., 2023 Nature Comm - read the paper
Proudly funded by
-
LRRK2 Investigative Therapeutics Exchange (LITE) - Michael J Fox Foundation
Jan 2025-present
Proudly part of the global consortium targeting therapeutics for treating Parkinson’s Disease
-
R01 - NIH/NHBLI
Dec 2022-present
Defining PRC2 complex epigenomic control in alveolar progenitor cells
-
R01 - NIH/NHBLI
Co-investigator with CCHMC Debora Sinner May 2021-present
Epigenetic Regulation of the Maturation and Function of Lung Epithelium by the SWI/SNF Proteins ARID1A and ARID1B
-
R01 - NIH/NHBLI - Co-investigator
Grant collaboration with CCHMC Whitsett Lab July 2022-present
PRDM3/16 Regulates Chromatin Accessibility to Determine Alveolar Maturation
-
UO1 - NIH/NIAID - Co-investigator
Grant collaboration with CCHMC Marazzi/Miraldi Labs Aug 2020-May 2025
Dynamic regulatory network models of human response to influenza virus
-
K08 - NIH/NHLBI
July 2018-June 2023
Mechanistic evaluation of a novel Wnt-responsive adult alveolar epithelial progenitor population during regeneration after diffuse alveolar damage.
-
CCHMC Procter Scholar Award
Jan 2021-Jan 2023
Combined activity of the PRC2 and BAF complexes define the regenerative capacity of alveolar progenitors
-
LUNGMAP2 Consortium Pilot and Feasibility Project
Aug 2021-July 2022
Integrating Epigenomic Analysis in a Rhesus Model of Developmental Lung Injury
-
Cystic Fibrosis Foundation Research and Development Program Pilot and Feasibility Studies
July 2019-June 2021
Mechanistic evaluation of the role of CFTR in alveolar regeneration
-
P30 - NIAMS
Parent Award (Thompson), Internal Pilot Project Award (Zacharias) July 2019-June 2021
Epigenomic Analysis of Lung Progenitor Cell Biology During Regeneration